Abstract
Introduction:
North American-ATLL (NA-ATLL) is a distinct genomic and clinical entity when compared with Japanese ATLL. Despite the frequent CNS involvement by this entity there are no prognostication tools to identify variables associated with higher risk. We studied one of the largest NA-ATLL cohorts in the United States at a minority rich ethnically diverse New York City based tertiary care center to identify discrete variables that may be associated with CNS involvement. Additionally, we examined the predictive power of the previously validated International Prognostic Index (IPI) developed for Diffuse Large B-cell Lymphoma (DLBCL) in terms of CNS involvement as well as overall survival in our cohort.
In our previous work, we assigned CNS involvement of ATLL if: 1) cerebrospinal fluid (CSF) cytology or flow cytometry was positive; 2) CNS imaging was positive for disease involvement or 3) physical exam was compatible with neurologic involvement.
Methods and results:
Of 65 NA-ATLL patients (pts), 20 (30.77%) had CNS involvement by ATLL meeting above mentioned criteria. The median age of all pts in our dataset was 59 years. Pts were divided into non- CNS involved NA-ATLL (non-CNS NA-ATLL) and CNS involved NA-ATLL (CNS NA-ATLL) groups. Parameters to predict CNS involvement were outlined as leukocytosis (>11000 per microliter [μL]), elevated acute lymphocytic count (>4000/ μL), elevated lactate dehydrogenase (LDH) (>190 units/liter [u/l]), generalized lymphadenopathy (LAD) defined as involvement of 2 or more non-contiguous nodal sites, elevated corrected serum calcium levels (>10.5 mg/deciliter), bone marrow (bm) involvement and serosal involvement defined as pleural or peritoneal involvement. These variables were chosen given our prior work identifying them as critical to predict OS in our general NA-ATLL population.
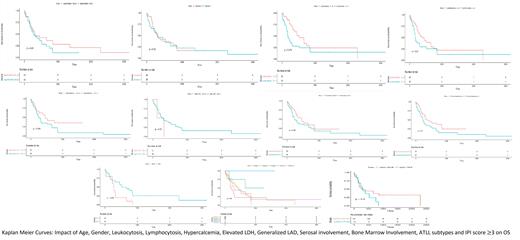
We then performed the Fisher test of proportions to determine if the variables above mentioned were different between the CNS NA-ATLL and the non-CNS NA-ATLL. We did not find significant associations when analyzing gender (p=0.0535), leukocytosis (p= 0.4001), lymphocytosis (p =0.57), hypercalcemia (p= 0.7661), elevated LDH (p= 0.4898), generalized LAD (p=0.271), serosal involvement (p= 0.05088) or bone marrow involvement (p=1). We only found a strong association when examining ATLL subtypes, being the acute/lymphomatous variant strongly associated with CNS involvement (p=2.226e10 -5).
To identify variables that can have an impact in OS in CNS NA-ATLL vs non-CNS NA-ATLL we calculated Kaplan Meier survival curves. We did not find any difference based on age (p=0.23), gender (p=0.95), leukocytosis (p= 0.074), lymphocytosis (p =0.21), hypercalcemia (p= 0.094), elevated LDH (p= 0.37), generalized LAD (p=0.28), and serosal involvement (p= 0.1) in the overall survival. The only significant association we found was bone marrow involvement in predicting poor OS in CNS NA-ATLL group (p= 0.038).
Next, we determined IPI scores (age>60, ECOG performance status>2, Ann Arbor stage III/IV, serum LDH score >190 u/L and more than 1 extra-nodal site) in our CNS NA ATLL and non-CNS NA ATLL cohorts. We sought to determine if: 1) this prognostication tool is predictive in NA-ATLL and 2) if these variables can be used to identify CNS involvement in our cohort.
CNS pts with a higher IPI score (>3) showed tendency towards a lower survival rate (p=0.089) than non-CNS pts (p=0.3). IPI score of 3 or more was not predictive of CNS involvement in our sample (p=1).
Conclusions:
NA-ATLL is a rare and protean disease with poor prognosis and CNS involvement is prevalent. With the variables used we did not find clear predictors of CNS associated disease other than patients presented with the lymphomatous and acute subtypes. We also observed than bone marrow involvement portends a dismal prognosis for individuals with CNS involvement. Future studies with a larger cohort could provider further insight on discovering predictors for CNS involvement in NA ATLL.
Shah: Celgene/BMS: Research Funding; Janssen: Research Funding. Gritsman: iOnctura: Research Funding. Shastri: GLC: Consultancy; Kymera Therapeutics: Research Funding; Guidepoint: Consultancy; Onclive: Honoraria. Verma: BMS: Research Funding; GSK: Research Funding; Incyte: Research Funding; Medpacto: Research Funding; Curis: Research Funding; Eli Lilly: Research Funding; Stelexis: Consultancy, Current equity holder in publicly-traded company; Novartis: Consultancy; Acceleron: Consultancy; Celgene: Consultancy; Stelexis: Current equity holder in publicly-traded company; Throws Exception: Current equity holder in publicly-traded company. Janakiram: Amgen: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal